Potential ALS Treatment Emerges from Nerve Cells in a Dish
When Hynek Wichterle, PhD, began studying stem cells back in 2000, any clinical application of his work was far from his mind. “As a postdoc at Columbia, I was interested in learning how a pluripotent stem cell, which has the potential to develop into any cell type in our body, becomes one type of neuron or another. It was pure developmental biology, as basic as research gets,” Wichterle says.
But today, Wichterle and his colleagues are tantalizingly close to bringing a new drug for amyotrophic lateral sclerosis (ALS) to market. If clinical testing, which started in March of this year, proves successful, the drug—prosetin—could be the first to directly target the neurodegenerative process in this invariably deadly neuromuscular disease.
Unlimited access to motor neurons sparks effort to find ALS therapy

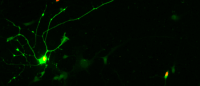
The creation of human motor neurons in the laboratory from stem cells was essential to the development of an experimental drug for ALS now in clinical testing. Image from Takazawa, et al. (2012) PloS One.
Wichterle’s unlikely journey from basic scientist to drug developer began when he discovered how to efficiently create functional spinal motor neurons in a laboratory dish from embryonic stem cells.
Motor neurons are the cells killed during ALS, and learning how to keep them alive could stop the disease.
But Wichterle’s initial interest in creating motor neurons in a dish was to study the blueprint of the nervous system, to decode mechanisms that control the construction of the most complex organ in our body. When he published his method nearly 20 years ago, “for the first time, we had unlimited access to specific types of neurons, allowing us to do all sorts of laboratory studies that weren’t possible before,” says Wichterle, now professor of pathology & cell biology, neuroscience (in neurology), and rehabilitation & regenerative medicine at Columbia University Vagelos College of Physicians and Surgeons and co-director of the Columbia University Motor Neuron Center.
Wichterle also realized that his technique could be used to study ALS and other motor neuron diseases and potentially find new treatments. He took his findings to Project ALS, then a new nonprofit organization, which agreed to support Wichterle’s research.
First drug candidates
Wichterle’s technique allowed ALS researchers to create motor neurons in the laboratory, but only healthy motor neurons. The researchers also needed to create neurons with the disease. In 2008, Wichterle, working with Chris Henderson, then at Columbia’s Motor Neuron Center, and Kevin Eggan at the Harvard Stem Cell Institute, learned how to reprogram skin cells from ALS patients into stem cells and then coax the cells into becoming motor neurons.
“It was a major breakthrough, but there was another challenge,” says Wichterle. “ALS usually develops between the ages of 55 and 75, decades after the neurons are first formed. We needed to find a way to accelerate the disease process so we could study neurons in the throes of ALS.”
Members of Wichterle’s lab sorted through more than a thousand chemical compounds to find one that killed neurons in a dish in a similar way as the disease, creating some of the same destructive changes found in the neurons of ALS patients in the process.
Using this new laboratory model of ALS, postdoctoral fellows Emily Lowry and Sebastian Thams in collaboration with Brent Stockwell’s lab in the chemistry and biological sciences departments at Columbia, identified a few compounds that stopped the neurodegenerative process and prevented death of the stressed ALS neurons.
All the compounds inhibited enzymes called MAP kinases, which tell stressed neurons to die.

Adding prosetin to motor neurons in the laboratory prevented the neurons from dying. Images: Wichterle laboratory, Columbia University Irving Medical Center.
“We saw a remarkable increase in motor neuron survival, it was really black and white, and this made me excited to continue testing this class of compounds,” Wichterle says. “The compounds essentially tell the neurons that they don’t have to die.”
Unfortunately, these initial compounds turned out to have little clinical value–they were insoluble, unstable, or unable to cross the blood-brain barrier.
Better clinical candidates through chemistry
Wichterle and Stockwell discussed the struggles with MAP kinase inhibitors with Arie Zask, a medicinal chemist who works with Stockwell and runs the Chemical Probe Synthesis Facility. Together, they decided to try to improve the pharmacological properties of the most promising neuroprotective compound. Aided by computer models that predicted how the compound interacts with its target in the cell, Pieter Bos, a postdoc in the Stockwell lab, tweaked the molecule and steadily improved the compound’s ability to persist in the body and engage its intended target.
Perhaps the greatest challenge was getting the compound across the blood-brain-barrier, which protects the central nervous system against toxins and pathogens. All told, the team went through more than 60 iterations of the compound over more than four years.
“By 2018, we had a compound—which we called prosetin—that was highly brain penetrant, highly potent, and highly selective for the enzyme we wanted to inhibit. We could always improve it further, but it was ready for testing in animals,” says Stockwell, professor of biological sciences and chemistry at Columbia University.
Those first experiments in a mouse model of ALS were encouraging.
“Prosetin did not extend lifespan, which was not all that surprising since we were using mice with an accelerated and aggressive form of the disease,” says Wichterle, “but the drug significantly delayed the animals’ weight loss and loss of grip strength, two key indicators of disease onset and progression.” These results together with a rigorous evaluation of the drug safety profile convinced the Food and Drug Administration to approve phase 1 clinical trials, which are designed to test a drug’s safety, tolerability, and dosing.
From mice to human clinical trials
Earlier this year, Project ALS teamed up with the Columbia group to found a new company called ProJenX, which raised funds to conduct the trials. As of this writing, 30 healthy volunteers have received prosetin. Testing in people with ALS may begin as early as this fall.
“For someone who still considers himself a basic scientist, this has been a fascinating journey,” says Wichterle. “But I want to emphasize that it’s just the beginning. It’s exciting that prosetin is in clinical trials, but it targets one aspect of the disease. ALS is complex, and we need to develop other drugs that can work in concert with each other to attack the disease on multiple fronts.”
The odds against prosetin are still long. Fewer than one in 10 drugs tested in phase 1 clinical trials are approved for use.
“I’ve worked on several potential drugs, and this is the first that has made it to clinical trials,” adds Stockwell. “We don't know if it's going to work in patients, but if it does, how amazing would that be?”
