Critical Flaw Found in Lab Models of the Human Blood-Brain Barrier
Cells used to study the human blood brain barrier in the lab aren’t what they seem, throwing nearly a decade’s worth of research into question, a new study from scientists at Columbia University Vagelos College of Physicians and Surgeons and Weill Cornell Medicine suggests.
The team also discovered a possible way to correct the error, raising hopes of creating a more accurate model of the human blood-brain barrier for studying certain neurological diseases and developing drugs that can cross it.
The study was published online Feb. 4 in the Proceedings of the National Academy of Sciences (PNAS).
“The blood-brain barrier is difficult to study in humans and there are many differences between the human and animal blood-brain barrier. So it’s very helpful to have a model of the human blood-brain barrier in a dish,” says co-study leader Dritan Agalliu, PhD, associate professor of pathology & cell biology (in neurology) at Columbia University Vagelos College of Physicians and Surgeons.
The in vitro human blood-brain barrier model, developed in 2012, is made by coaxing differentiated adult cells, such as skin cells, into stem cells that behave like embryonic stem cells. These induced pluripotent stem cells can then be transformed into mature cells of almost any type—including a type of endothelial cell that lines the blood vessels of the brain and spinal cord and forms a unique barrier that normally restricts the entry of potentially dangerous substances, antibodies, and immune cells from the bloodstream into the brain.

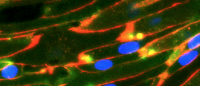
Left image shows misidentified cells from an in vitro model of the human blood-brain barrier; right image shows cells that have been reprogrammed to more closely resemble endothelial cells of the human blood-brain barrier. (Source: Agalliu lab, Columbia University Vagelos College of Physicians and Surgeons)
Agalliu previously noticed that these induced human “brain microvascular endothelial cells,” produced using the published approach in 2012, did not behave like normal endothelial cells in the human brain. “This raised my suspicion that the protocol for making the barrier’s endothelial cells may have generated cells of the wrong identity,” says Agalliu.
“At the same time the Weill Cornell Medicine team had similar suspicions, so we teamed up to reproduce the protocol and perform bulk and single-cell RNA sequencing of these cells.”
Their analysis revealed that the supposed human brain endothelial cells were missing several key proteins found in natural endothelial cells and had more in common with a completely different type of cell (epithelial) that is normally not found in the brain.
The team also identified three genes that, when activated within induced pluripotent cells, lead to the creation of cells that behave more like bona fide endothelial cells. More work is still needed, Agalliu says, to create endothelial cells that produce a reliable model of the human blood-brain barrier. His team is working to address this problem.
“The misidentification of human brain endothelial cells may be an issue for other types of cells made from induced pluripotent cells such as astrocytes or pericytes that form the neurovascular unit,” Agalliu says. The protocols to generate these cells were created before the advent of single-cell technologies that are better at uncovering a cell’s identity. “Cell misidentification remains a major problem that needs to be addressed in the scientific community in order to develop cells that mirror those found in the human brain. This will allow us to use these cells to study the role of genetic risk factors for neurological disorders and develop drug therapies that target the correct cells that contribute to the blood-brain barrier.”
References
More information
The study is titled “Pluripotent stem cell-derived epithelium misidentified as brain microvascular endothelium requires ETS factors to acquire vascular fate.”
The other contributors: Tyler M. Lu (Weill Cornell Medicine), Sean Houghton (Weill Cornell Medicine), Tarig Magdeldin (Weill Cornell Medicine), José Gabriel Barcia Durán (Weill Cornell Medicine), Andrew P. Minotti (Weill Cornell Medicine), Amanda Snead (Columbia), Andrew Sproul (Columbia), Duc-Huy T. Nguyen (Weill Cornell Medicine), Jenny Xiangh (Weill Cornell Medicine), Howard A. Fine (Weill Cornell Medicine), Zev Rosenwaks (Weill Cornell Medicine), Lorenz Studer (Memorial Sloan Kettering Cancer Center and Weill Cornell Medicine), Shahin Rafii (Weill Cornell Medicine), David Redmond (Weill Cornell Medicine), and Raphaël Lis (Weill Cornell Medicine).
The study was supported by the National Institutes of Health (grants R01MH112849, R01NS107344, RF1AG054023, DP1CA228040, and RF1AG054023), the Leducq Foundation, John Castle (Newport Equity LLC), the PANDAS Network, the Thompson Foundation, the Henry and Marilyn Taub Foundation, NYSTEM, the Ansary Stem Cell Institute, and the Starr Foundation TRI-Institution Stem Cell Initiative.
The authors report no financial or other conflicts of interest.